
Claiming that "acetaminophen may cause autism," Trump warns pregnant women to avoid taking Tylenol

美國總統特朗普警告孕婦避免服用泰諾,稱其成分對乙酰氨基酚可能與自閉症有關,儘管缺乏廣泛認可的科學證據。特朗普建議女性在懷孕期間限制泰諾使用,除非醫學上必需。美國政府計劃修改藥品安全標籤並展開公共衞生宣傳。泰諾製造商 Kenvue 表示,科學研究表明對乙酰氨基酚不會導致自閉症。股價因特朗普言論重挫近 7.5%。科學界對此存在爭議,部分專家認為不應因恐懼放棄該藥的益處。
美國總統特朗普警告孕婦避免服用泰諾,稱其主要成分對乙酰氨基酚與自閉症存在關聯,儘管這一風險缺乏廣泛認可的科學證據支持。
9 月 22 日,特朗普在與美國衞生部長肯尼迪的聯合活動中表示:
服用泰諾不好,我會直説這不好。基於這個原因,強烈建議女性除非醫學上必需,在懷孕期間限制泰諾的使用。
美國政府同時宣佈,計劃啓動程序修改相關藥品安全標籤,並展開全國性的公共衞生宣傳。
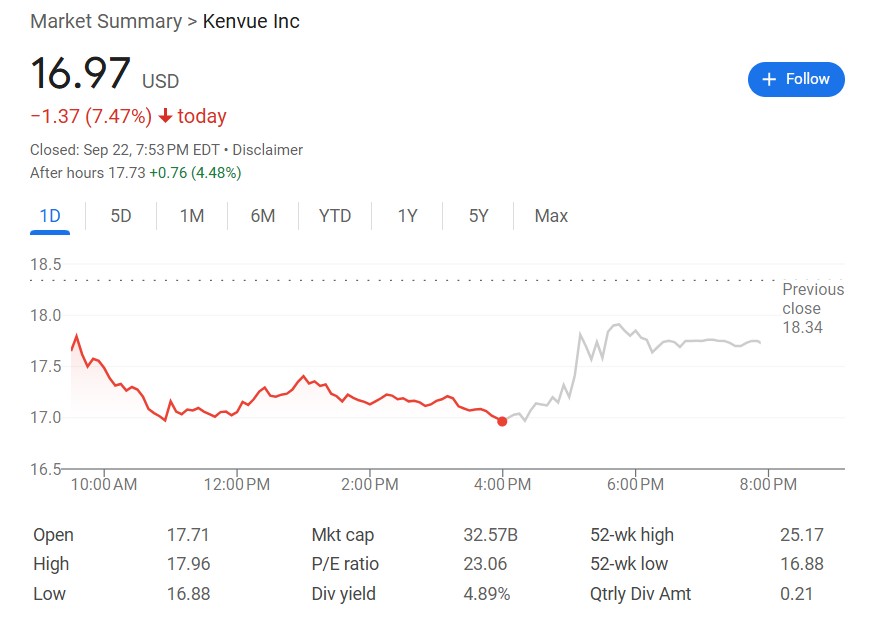
泰諾製造商 Kenvue 聲明稱"獨立、可靠的科學研究"表明服用對乙酰氨基酚不會導致自閉症。週一公司股價重挫近 7.5%、收於歷史低點,但盤後交易中反彈超 4.48%。
科學界爭議與現有證據
對乙酰氨基酚常被推薦給孕婦作為替代布洛芬的退燒藥。過去十餘年,關於對乙酰氨基酚與自閉症之間關聯的研究時有出現,但結論不一。
在藥學中,對乙酰氨基酚可以歸為 “解熱鎮痛藥”,不僅有退熱的作用,還有一定的鎮痛作用。既可以退熱,還能緩解肌肉痠痛的症狀,所以最常被添加到泰諾及市面上多種非處方感冒退燒藥中。
雖然2021 年《自然內分泌學綜述》上的一篇論文呼籲謹慎使用,但一項於2024 年發表、分析了瑞典近 250 萬名同胞兄弟姐妹數據的大規模研究發現,當母親在孕期服用對乙酰氨基酚後,後代患自閉症的風險並未增加。
與此同時,美國婦產科醫師學會等多個專業團體對此提出異議,認為 “不應讓患者因恐懼而放棄對乙酰氨基酚的諸多益處”。
一些科學家也警告稱,在沒有確鑿證據的情況下讓孕婦停用該藥為時過早,因為該藥是孕婦在懷孕期間某些階段唯一推薦使用的退燒藥。他們表示,長時間不治療的發燒本身可能會損害胎兒發育。
美國食品藥品監督管理局(FDA)則在上月更新的官方網站信息中指出,該機構 “尚未發現明確證據表明,在懷孕期間適當使用對乙酰氨基酚會導致不良的妊娠、出生、神經行為或發育後果”。
法律糾紛與監管風向
在特朗普政府發出警告之前,相關爭議已進入司法程序。
此前,美國有大量訴訟稱,孕期接觸非處方止痛藥泰諾導致了兒童自閉症。
但在 2023 年,美國地區法官 Denise Cote 駁回了這些訴訟背後的科學證據,稱其基於 “有缺陷的科學”。她還排除了原告方專家證人 Andrea Baccarelli 的證詞,認為其在衡量對乙酰氨基酚與神經發育障礙關聯時未採用 “足夠的嚴謹性”。
目前,原告方已提起上訴,預計今年晚些時候將在曼哈頓進行辯論。法律專家表示,衞生與公眾服務部(HHS)關於自閉症的聲明可能會被上訴法官考慮,但無法取代關於可採納證據的法律檢驗標準。
與此同時,美國衞生部長肯尼迪已明確表示,FDA 將就懷孕期間使用對乙酰氨基酚的風險發佈醫師通知,並啓動更改藥品安全標籤的程序。
這預示着,儘管美國司法層面存在爭議,但行政監管的壓力正在加大。
風險提示及免責條款
市場有風險,投資需謹慎。本文不構成個人投資建議,也未考慮到個別用户特殊的投資目標、財務狀況或需要。用户應考慮本文中的任何意見、觀點或結論是否符合其特定狀況。據此投資,責任自負。

